Understanding the CO2-pH Chart: A Complete Information to Carbon Dioxide’s Affect on Aquatic Methods
Associated Articles: Understanding the CO2-pH Chart: A Complete Information to Carbon Dioxide’s Affect on Aquatic Methods
Introduction
With nice pleasure, we are going to discover the intriguing subject associated to Understanding the CO2-pH Chart: A Complete Information to Carbon Dioxide’s Affect on Aquatic Methods. Let’s weave attention-grabbing info and supply recent views to the readers.
Desk of Content material
Understanding the CO2-pH Chart: A Complete Information to Carbon Dioxide’s Affect on Aquatic Methods
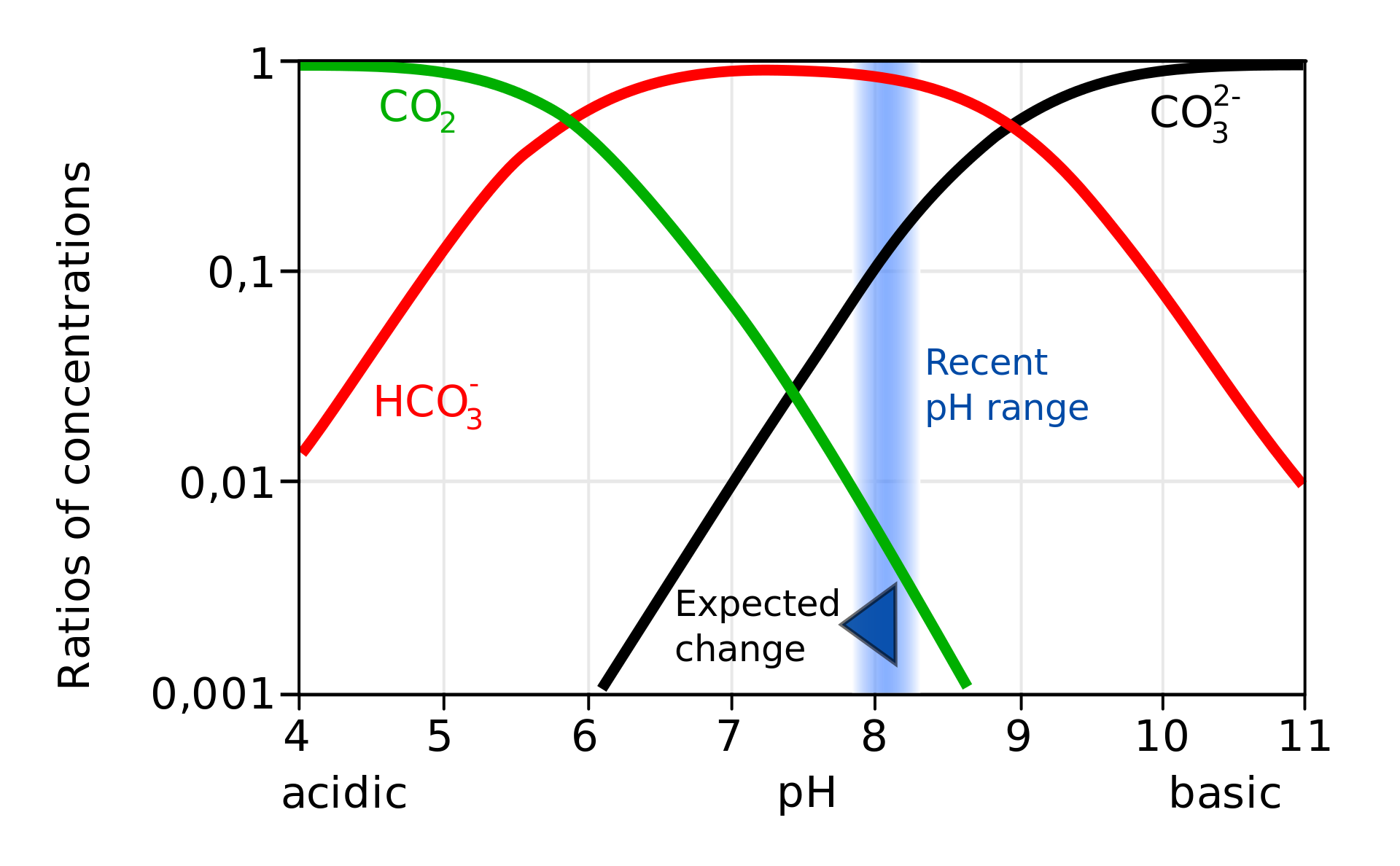
Carbon dioxide (CO2), a ubiquitous fuel within the Earth’s ambiance, performs an important function in regulating the pH of varied aquatic methods, from oceans and lakes to smaller our bodies of water. The connection between CO2 and pH is advanced however basically essential for understanding aquatic ecosystems’ well being and stability. This text delves into the intricacies of the CO2-pH chart, explaining its building, interpretation, and implications for environmental science, aquaculture, and different fields.
The Chemistry Behind the CO2-pH Relationship:
The connection between CO2 and pH stems from CO2’s skill to react with water to kind carbonic acid (H2CO3), a weak acid. This response is reversible:
CO2 (g) + H2O (l) ⇌ H2CO3 (aq)
Carbonic acid then partially dissociates into bicarbonate ions (HCO3-) and hydrogen ions (H+):
H2CO3 (aq) ⇌ H+ (aq) + HCO3- (aq)
The focus of hydrogen ions (H+) instantly determines the pH of the answer. A better focus of H+ signifies a decrease pH (extra acidic), whereas a decrease focus of H+ signifies the next pH (extra alkaline or primary). Subsequently, a rise in atmospheric CO2 results in a rise in carbonic acid formation, leading to the next focus of H+ and consequently a decrease pH. This course of is called ocean acidification when utilized to the world’s oceans.
Establishing the CO2-pH Chart:
A CO2-pH chart, additionally typically referred to as a CO2-carbonate system diagram, graphically represents the connection between the partial stress of CO2 (pCO2, normally expressed in atmospheres or µatm), pH, and the concentrations of varied carbonate species (HCO3-, CO32-, H2CO3) in an aqueous answer. These charts are sometimes constructed utilizing equilibrium constants (K1 and K2 for the dissociation of carbonic acid) and the legislation of mass motion. The precise form and values on the chart depend upon a number of components, together with:
- Temperature: The equilibrium constants for carbonic acid dissociation are temperature-dependent. Larger temperatures typically shift the equilibrium in the direction of decrease pH for a given pCO2.
- Salinity: Salinity influences the exercise coefficients of ions, affecting the obvious equilibrium constants and thus the connection between pCO2 and pH. Seawater, with its increased salinity, will exhibit a distinct CO2-pH relationship in comparison with freshwater.
- Alkalinity: Alkalinity, a measure of the water’s capability to withstand modifications in pH, considerably impacts the chart. Larger alkalinity buffers the system in opposition to pH modifications attributable to variations in pCO2.
The chart normally shows curves representing totally different whole alkalinity ranges. For a given pCO2, the intersection of the pCO2 line and the suitable alkalinity curve reveals the corresponding pH. The chart additionally usually contains traces representing the concentrations of varied carbonate species, permitting for a complete understanding of the carbonate system’s composition.
Deciphering the CO2-pH Chart:
The CO2-pH chart offers precious insights into a number of elements of aquatic methods:
- Acidification tendencies: By evaluating historic and present pCO2 ranges, the chart can be utilized to visualise the extent of ocean acidification or modifications in pH inside lakes or rivers attributable to elevated CO2 emissions.
- Buffering capability: The spacing between alkalinity curves signifies the system’s buffering capability. Intently spaced curves counsel a decrease buffering capability, that means that small modifications in pCO2 result in important pH modifications. Broadly spaced curves point out the next buffering capability, the place pH stays comparatively steady regardless of fluctuations in pCO2.
- Carbonate species distribution: The chart reveals how the relative proportions of dissolved CO2, H2CO3, HCO3-, and CO32- change with various pCO2 and pH. This info is essential for understanding the provision of carbonate ions for shell formation in marine organisms like corals and shellfish.
- Predicting future modifications: By modeling future pCO2 eventualities based mostly on local weather change projections, the chart can be utilized to foretell the potential influence on aquatic system pH and the distribution of carbonate species. That is very important for assessing the dangers to marine ecosystems and biodiversity.
Purposes of the CO2-pH Chart:
The CO2-pH chart finds purposes in quite a few fields:
- Oceanography: Understanding ocean acidification and its influence on marine life is essential. The chart helps researchers mannequin and predict future ocean pH modifications and their penalties for marine ecosystems.
- Limnology: The chart is crucial for finding out the chemistry of lakes and rivers, serving to researchers perceive the results of CO2 inputs from numerous sources (e.g., atmospheric deposition, runoff) on water high quality and aquatic life.
- Aquaculture: Sustaining optimum pH ranges is important for profitable aquaculture. The chart assists in managing CO2 ranges and water chemistry in aquaculture methods to make sure the well being and productiveness of farmed species.
- Environmental monitoring: The chart aids in monitoring water high quality by offering a transparent illustration of the connection between CO2 and pH. This enables for early detection of potential issues and informs efficient administration methods.
- Geochemistry: The CO2-pH chart is utilized in finding out the geochemical processes in pure waters, together with the weathering of rocks and the transport of carbon in aquatic methods.
Limitations of the CO2-pH Chart:
Whereas extremely helpful, the CO2-pH chart has sure limitations:
- Simplified mannequin: The chart represents a simplified mannequin of the carbonate system, neglecting different components that may affect pH, such because the presence of different acids and bases, natural matter, and organic processes.
- Equilibrium assumption: The chart is predicated on equilibrium situations. In actuality, aquatic methods are sometimes not at equilibrium, and kinetic components can have an effect on the precise pH and carbonate species distribution.
- Knowledge enter accuracy: The accuracy of the chart relies on the accuracy of the enter knowledge, together with temperature, salinity, and alkalinity measurements.
Conclusion:
The CO2-pH chart is a robust device for understanding the intricate relationship between carbon dioxide and pH in aquatic methods. Its purposes vary from predicting the impacts of local weather change on oceans to optimizing water high quality in aquaculture. Whereas simplified, the chart offers a precious framework for researchers, environmental managers, and anybody within the well being and sustainability of aquatic environments. By appreciating each its strengths and limitations, we will leverage this device to achieve essential insights into the advanced chemistry of our planet’s water our bodies and develop efficient methods for his or her safety.





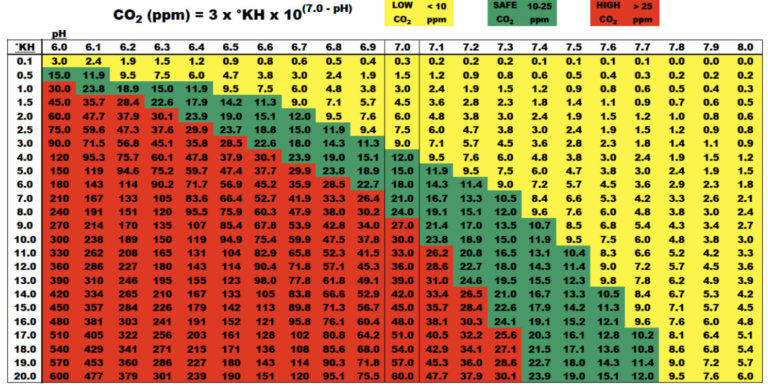

Closure
Thus, we hope this text has offered precious insights into Understanding the CO2-pH Chart: A Complete Information to Carbon Dioxide’s Affect on Aquatic Methods. We respect your consideration to our article. See you in our subsequent article!