Decoding the Colours of the pH Chart: A Complete Information to Understanding Acidity and Alkalinity
Associated Articles: Decoding the Colours of the pH Chart: A Complete Information to Understanding Acidity and Alkalinity
Introduction
On this auspicious event, we’re delighted to delve into the intriguing subject associated to Decoding the Colours of the pH Chart: A Complete Information to Understanding Acidity and Alkalinity. Let’s weave attention-grabbing data and provide contemporary views to the readers.
Desk of Content material
Decoding the Colours of the pH Chart: A Complete Information to Understanding Acidity and Alkalinity

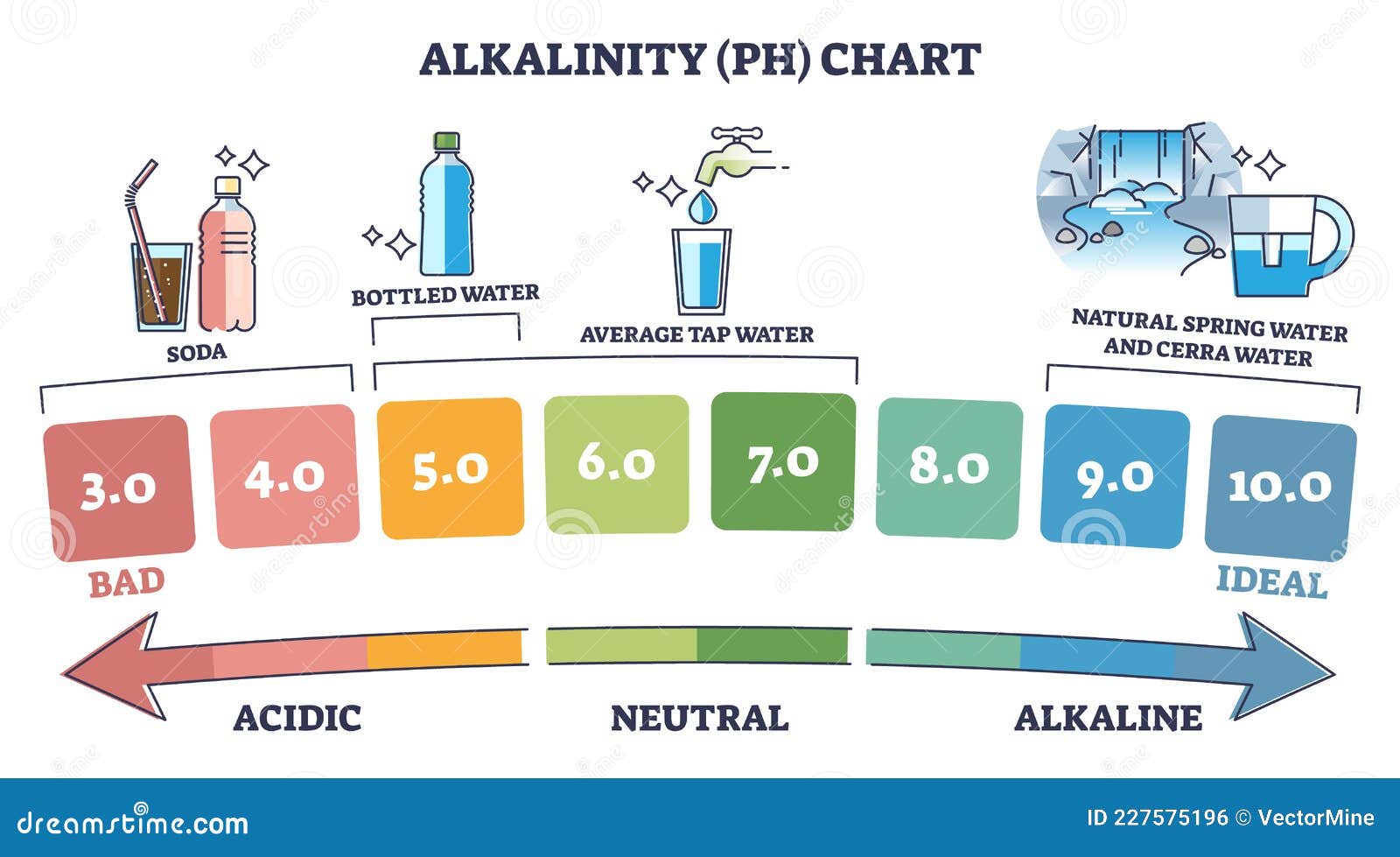
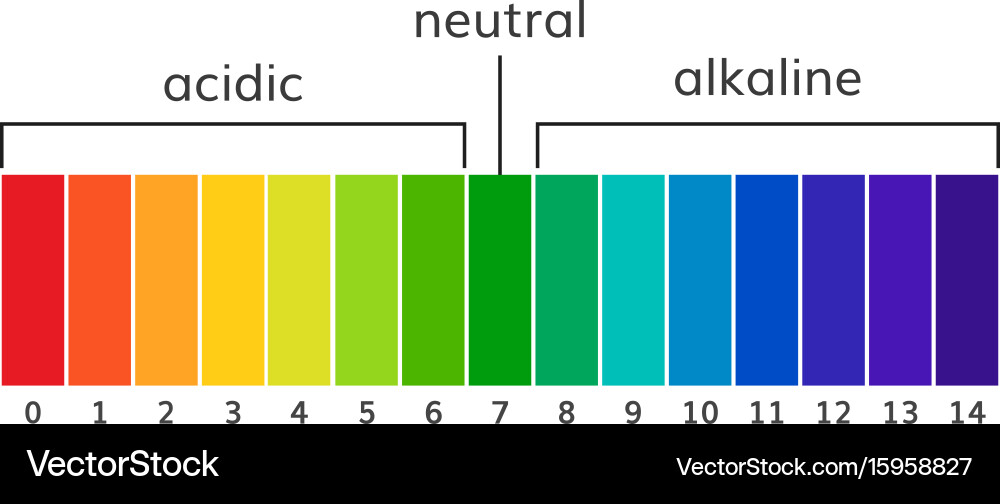
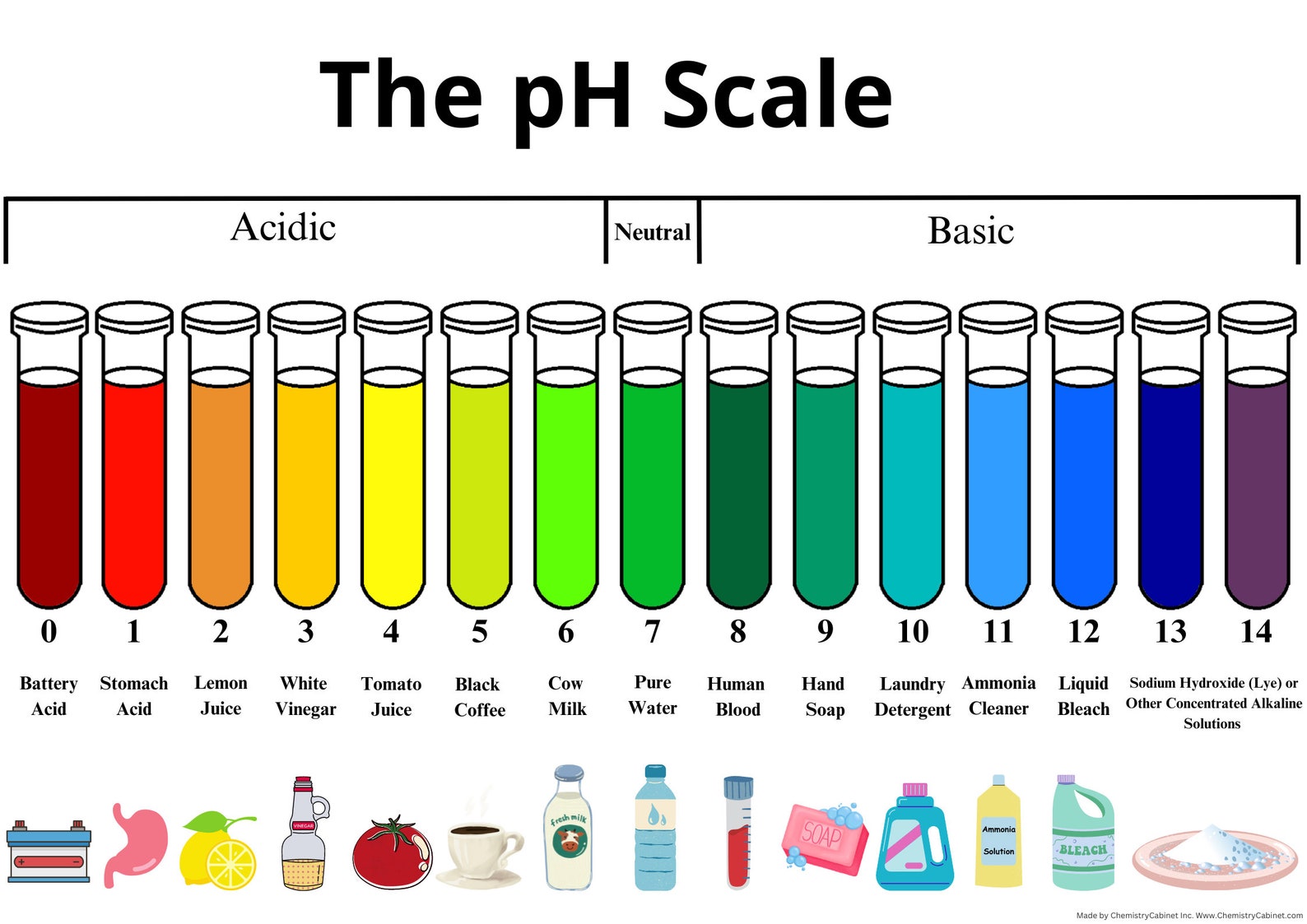
The pH scale, a logarithmic scale starting from 0 to 14, is a basic idea in chemistry and quite a few different fields, from agriculture and environmental science to medication and meals processing. It measures the focus of hydrogen ions (H⁺) in an answer, indicating its acidity or alkalinity. Whereas numerical values are exact, the visible illustration of the pH scale, typically depicted as a coloration chart, offers a readily comprehensible and intuitive strategy to grasp the relative acidity or alkalinity of a substance. This text delves into the nuances of pH chart colours, explaining their significance, the underlying chemistry, and the purposes of this significant instrument.
The Chemistry Behind the Colours:
The colour adjustments noticed in pH indicators, the substances used to create pH charts, are a results of particular chemical reactions. These indicators are usually weak acids or bases that exhibit completely different colours relying on the pH of their surroundings. The change in coloration shouldn’t be abrupt however moderately a gradual transition throughout a particular pH vary, generally known as the indicator’s transition vary. This transition vary is essential as a result of it determines the accuracy and reliability of the color-based pH willpower.
For instance, a standard indicator, phenolphthalein, is colorless in acidic options (pH under 8.2) and turns pink in alkaline options (pH above 10.0). The transition from colorless to pink happens step by step inside this pH vary. Different indicators, comparable to methyl orange (pink in acidic options, yellow in alkaline options) and bromothymol blue (yellow in acidic options, blue in alkaline options), have completely different transition ranges and thus completely different coloration adjustments. The cautious collection of indicators is important for creating correct and complete pH charts.
The Development of a pH Chart:
A typical pH chart shows a spectrum of colours comparable to completely different pH values. These colours are usually derived from a mixture of indicators, chosen to cowl your complete pH vary (0-14) with overlapping transition ranges to make sure correct coloration illustration throughout the spectrum. The chart typically consists of numerical pH values alongside the colour gradients, facilitating straightforward interpretation.
The creation of a pH chart entails cautious calibration and standardization. This course of entails utilizing options of identified pH values to find out the exact coloration related to every pH level. The colour accuracy is essential for dependable pH willpower utilizing the chart. Variations within the manufacturing course of, the focus of the indications, and even lighting situations can have an effect on the colour notion and therefore the accuracy of the chart.
Decoding the Colours:
The colours on a pH chart should not arbitrarily chosen; they signify a steady transition from acidic to impartial to alkaline situations. Typically:
-
Crimson and Orange: These colours signify strongly acidic situations (pH 0-3). The depth of the pink usually will increase with lowering pH, indicating increased acidity.
-
Yellow and Inexperienced: These colours signify a transition from acidic to impartial situations (pH 4-6). Yellow represents weakly acidic options, whereas inexperienced signifies a more in-depth method to neutrality.
-
Blue and Purple: These colours signify alkaline situations (pH 8-14). The depth of the blue or purple usually will increase with growing pH, indicating increased alkalinity.
-
Inexperienced (Impartial): A pure inexperienced coloration, typically positioned round pH 7, represents impartial situations the place the focus of hydrogen ions and hydroxide ions (OH⁻) is equal. That is the purpose of pure water at 25°C.
It is essential to keep in mind that the colour transitions are gradual, and the precise coloration at a particular pH may range barely relying on the particular indicators used within the chart’s creation. Due to this fact, it’s all the time advisable to make use of the numerical pH values alongside the colour for essentially the most correct willpower.
Purposes of pH Charts and Shade Indicators:
pH charts and coloration indicators discover widespread purposes throughout varied fields:
-
Agriculture: Soil pH is essential for plant progress. Farmers use pH charts to find out soil acidity and regulate it accordingly by including lime (to extend pH) or different amendments (to lower pH). This ensures optimum nutrient availability and wholesome plant growth.
-
Aquaculture: Sustaining the right pH in aquariums and fish ponds is important for the well being and survival of aquatic organisms. Shade indicators present a fast and straightforward strategy to monitor pH ranges and make needed changes.
-
Environmental Monitoring: Water high quality evaluation typically entails pH measurement. pH charts are used to find out the acidity or alkalinity of water our bodies, serving to to determine air pollution sources and assess the general well being of the ecosystem.
-
Meals and Beverage Trade: pH management is important in meals processing, influencing style, texture, and preservation. Shade indicators assist monitor pH ranges throughout varied levels of meals manufacturing, guaranteeing product high quality and security.
-
Medication: pH ranges within the physique are intently regulated. Shade indicators can be utilized in medical diagnostics to evaluate the acidity or alkalinity of bodily fluids, comparable to urine or blood, aiding in illness analysis and therapy.
-
Swimming Swimming pools: Sustaining the right pH in swimming swimming pools is important for water security and hygiene. Common pH monitoring utilizing coloration indicators ensures a snug and secure swimming surroundings.
-
Chemistry Laboratories: pH charts and indicators are indispensable instruments in chemistry laboratories for varied experiments and analyses. They supply a fast and handy methodology for figuring out the pH of options and monitoring reactions.
Limitations of pH Charts:
Whereas pH charts are helpful instruments, they’ve limitations:
-
Subjectivity: Shade notion can range amongst people, resulting in slight inaccuracies in pH willpower. This may be influenced by lighting situations and particular person coloration imaginative and prescient.
-
Indicator Limitations: Every indicator has a particular transition vary. Utilizing a single indicator may not present correct outcomes throughout your complete pH spectrum. A mix of indicators is usually needed for complete pH willpower.
-
Interference: The presence of different substances within the resolution can intrude with the indicator’s coloration change, resulting in inaccurate readings. This necessitates cautious consideration of potential interferences.
-
Precision: pH charts usually present solely approximate pH values. For exact measurements, digital pH meters are needed.
Conclusion:
The pH chart, with its vibrant spectrum of colours, offers a readily accessible and visually intuitive strategy to perceive and assess the acidity or alkalinity of an answer. Whereas it presents a fast and handy methodology for approximate pH willpower, it is essential to know its limitations and use it along side different strategies, comparable to digital pH meters, for exact measurements. The widespread purposes of pH charts and coloration indicators in varied fields spotlight their significance as important instruments for monitoring and controlling pH ranges, guaranteeing high quality, security, and optimum situations in various purposes. Understanding the chemistry behind the colours and the constraints of the charts permits for his or her efficient and accountable use.




Closure
Thus, we hope this text has supplied invaluable insights into Decoding the Colours of the pH Chart: A Complete Information to Understanding Acidity and Alkalinity. We hope you discover this text informative and useful. See you in our subsequent article!