Decoding the Atom: A Complete Information to Electron Configuration Charts
Associated Articles: Decoding the Atom: A Complete Information to Electron Configuration Charts
Introduction
With nice pleasure, we are going to discover the intriguing subject associated to Decoding the Atom: A Complete Information to Electron Configuration Charts. Let’s weave attention-grabbing data and supply contemporary views to the readers.
Desk of Content material
Decoding the Atom: A Complete Information to Electron Configuration Charts
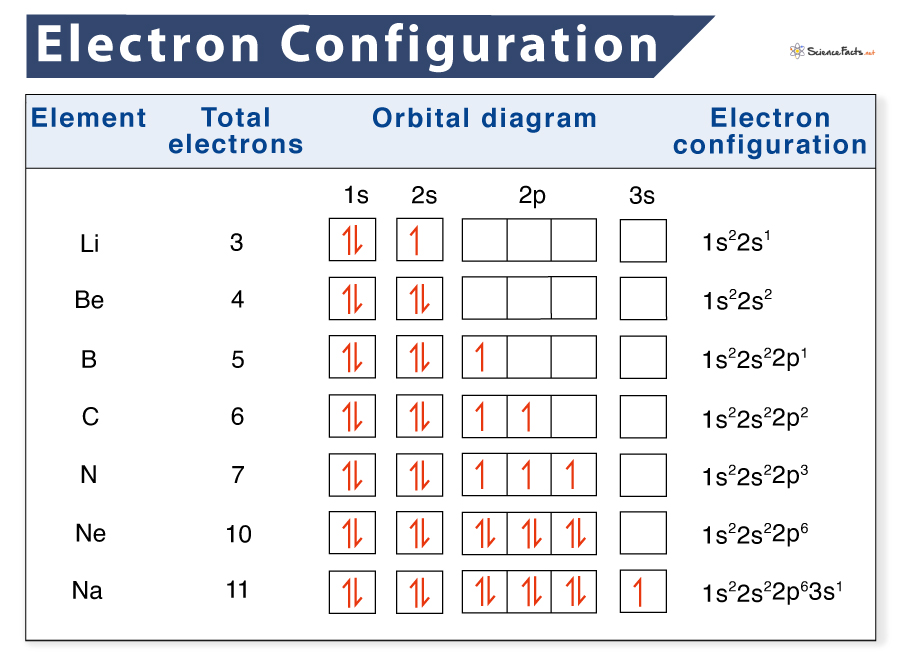
The atom, the elemental constructing block of matter, is a posh entity ruled by the legal guidelines of quantum mechanics. Understanding the association of electrons inside an atom is essential for comprehending its chemical properties, reactivity, and total conduct. This association is meticulously described by its electron configuration, a shorthand notation that visually represents the distribution of electrons among the many varied vitality ranges and sublevels throughout the atom. Electron configuration charts, subsequently, function important instruments for chemists, physicists, and anybody searching for a deeper understanding of atomic construction.
The Basis: Vitality Ranges and Sublevels
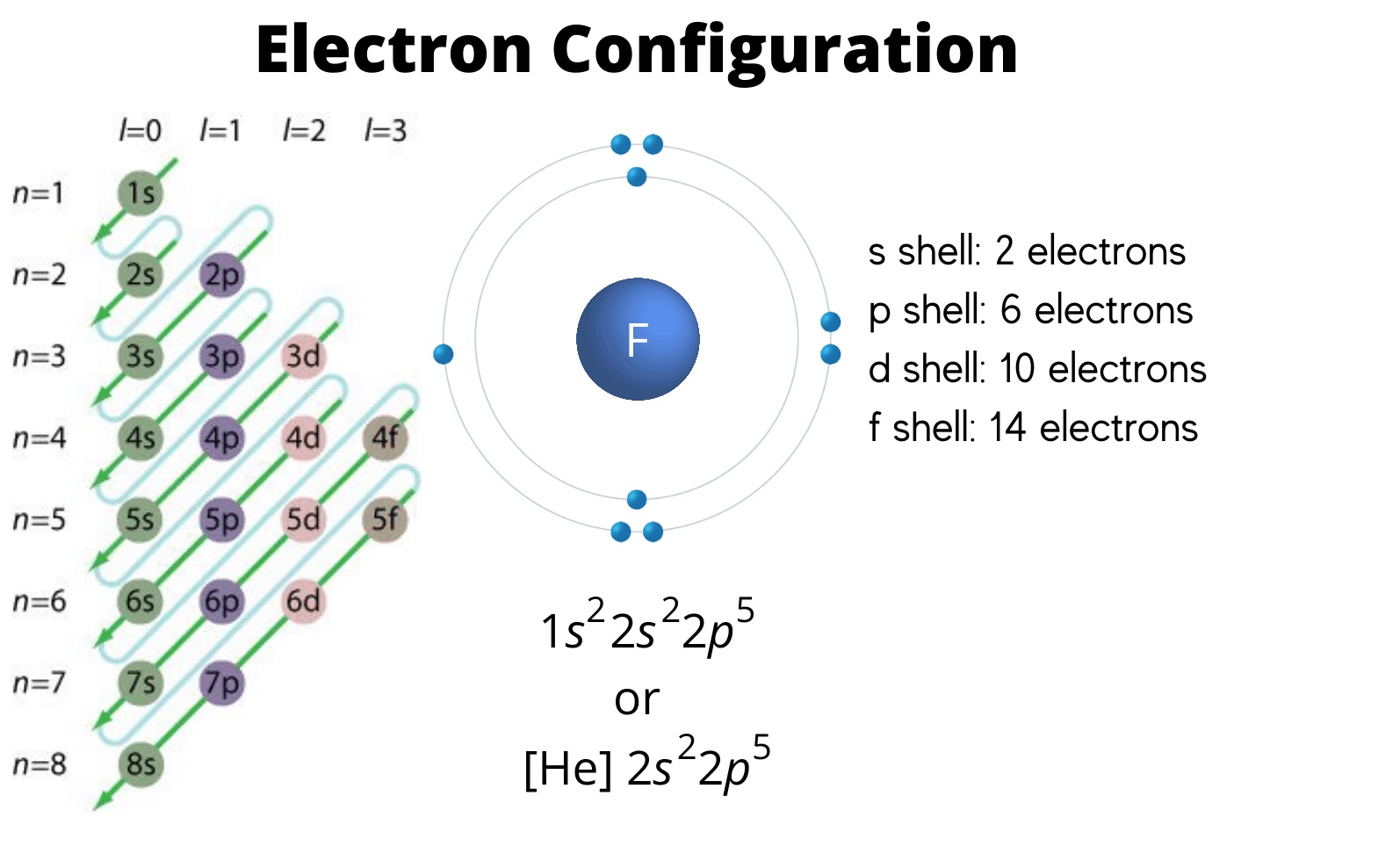
Earlier than delving into the intricacies of electron configuration charts, it is important to determine a foundational understanding of atomic construction. Electrons do not merely orbit the nucleus in random paths; they occupy particular vitality ranges, typically visualized as shells surrounding the nucleus. These vitality ranges are designated by principal quantum numbers (n), the place n = 1 represents the bottom vitality stage (closest to the nucleus), n = 2 the subsequent, and so forth. Every vitality stage can maintain a most variety of electrons, decided by the formulation 2n². Thus, the primary vitality stage (n=1) can maintain a most of two electrons, the second (n=2) 8, the third (n=3) 18, and so forth.
Nonetheless, the story would not finish with vitality ranges. Inside every vitality stage (besides the primary), there are sublevels, denoted by the letters s, p, d, and f. These sublevels symbolize totally different shapes and orientations of electron orbitals, areas of house the place there is a excessive chance of discovering an electron. Every sublevel can accommodate a particular variety of electrons:
- s sublevel: spherical in form, holds a most of two electrons.
- p sublevel: dumbbell-shaped, holds a most of 6 electrons (3 orbitals, 2 electrons per orbital).
- d sublevel: extra complicated shapes, holds a most of 10 electrons (5 orbitals, 2 electrons per orbital).
- f sublevel: much more complicated shapes, holds a most of 14 electrons (7 orbitals, 2 electrons per orbital).
Setting up Electron Configurations: The Aufbau Precept and Hund’s Rule
The method of assigning electrons to those vitality ranges and sublevels follows particular guidelines:
-
Aufbau Precept: Electrons fill the bottom vitality ranges first. This precept dictates a predictable order of filling, beginning with the bottom vitality stage (1s) and progressing to greater vitality ranges. The order is just not strictly sequential based mostly on the principal quantum quantity (n) as a result of delicate vitality variations between sublevels.
-
Hund’s Rule: Inside a sublevel, electrons will individually occupy every orbital earlier than pairing up. This minimizes electron-electron repulsion and results in a extra secure configuration. Every orbital inside a sublevel is first crammed with a single electron earlier than any pairing happens. These unpaired electrons have parallel spins, represented by arrows pointing in the identical course.
-
Pauli Exclusion Precept: No two electrons in an atom can have the identical set of 4 quantum numbers. Which means that every orbital can maintain a most of two electrons, with reverse spins (one spin up, one spin down).
Studying and Deciphering Electron Configuration Charts
Electron configuration charts sometimes symbolize the electron configuration utilizing a shorthand notation. For instance, the electron configuration of oxygen (atomic quantity 8) is written as 1s²2s²2p⁴. This notation signifies:
- 1s²: Two electrons within the 1s sublevel.
- 2s²: Two electrons within the 2s sublevel.
- 2p⁴: 4 electrons within the 2p sublevel.
The superscript numbers point out the variety of electrons in every sublevel. The order of filling follows the Aufbau precept, leading to a particular association of electrons for every factor.
Extra complicated components require a extra intensive notation, incorporating greater vitality ranges and sublevels. As an illustration, the electron configuration of iron (atomic quantity 26) is 1s²2s²2p⁶3s²3p⁶4s²3d⁶. Discover that the 4s sublevel fills earlier than the 3d sublevel, although the 3d sublevel has a decrease principal quantum quantity. That is as a result of delicate vitality variations between sublevels, a consequence of quantum mechanical interactions.
Exceptions to the Guidelines: The Irregularities
Whereas the Aufbau precept offers a normal framework for predicting electron configurations, there are exceptions. Some components deviate from the anticipated order of filling resulting from elements like elevated stability achieved by way of half-filled or fully stuffed sublevels. For instance, chromium (atomic quantity 24) and copper (atomic quantity 29) exhibit configurations that differ from the anticipated ones. These exceptions spotlight the complexities of electron-electron interactions and the inherent limitations of simplified fashions.
Purposes of Electron Configuration Charts
Electron configuration charts aren’t simply theoretical constructs; they’ve important sensible purposes throughout varied scientific fields:
-
Predicting Chemical Properties: The electron configuration straight influences a component’s chemical conduct. The outermost electrons, often known as valence electrons, are primarily answerable for chemical bonding and reactivity. Components with related valence electron configurations typically exhibit related chemical properties, resulting in the group of the periodic desk.
-
Spectroscopy: The association of electrons dictates the vitality ranges inside an atom. When an atom absorbs vitality, electrons can transition to greater vitality ranges. When these electrons return to their floor state, they emit vitality within the type of mild. The particular wavelengths of sunshine emitted are attribute of the factor and are utilized in spectroscopic methods for factor identification and evaluation.
-
Supplies Science: Understanding electron configurations is essential in designing and characterizing supplies with particular properties. The digital construction of supplies determines their electrical conductivity, magnetic properties, and optical conduct.
-
Nuclear Chemistry: Electron configurations are related in understanding the conduct of radioactive isotopes and nuclear reactions. The soundness of the nucleus is influenced by the digital construction of the atom.
Past the Fundamentals: Orbital Diagrams and Quantum Numbers
Whereas electron configuration notation offers a concise illustration of electron distribution, a extra detailed understanding requires using orbital diagrams and a deeper exploration of quantum numbers. Orbital diagrams visually symbolize the electrons in every orbital, utilizing arrows to point electron spin. This offers a clearer image of electron pairing and unpaired electrons. The 4 quantum numbers (principal, azimuthal, magnetic, and spin) present an entire description of an electron’s state inside an atom.
Conclusion:
Electron configuration charts are indispensable instruments for understanding atomic construction and chemical conduct. They supply a framework for predicting the properties of components and are important for varied scientific purposes. Whereas the Aufbau precept and Hund’s rule present a normal guideline, exceptions spotlight the complexities of electron-electron interactions inside atoms. An intensive understanding of electron configurations requires familiarity with orbital diagrams, quantum numbers, and the nuances of electron-electron interactions. Mastering this idea unlocks a deeper appreciation for the intricacies of the atomic world and its profound affect on the properties of matter.

![List of Electron Configuration Chart of All Elements [PDF]](https://iperiodictable.com/wp-content/uploads/2021/03/What-is-Electron-Configuration-768x431.png)


Closure
Thus, we hope this text has offered precious insights into Decoding the Atom: A Complete Information to Electron Configuration Charts. We thanks for taking the time to learn this text. See you in our subsequent article!